

By: Adriaan van Korlaar – XEAMOS
10 July 2019
What are particulates?
Particulates, which are also known as (diesel) soot, are a collection of carbon particles, hydrocarbons, ash residue from the lubricant, fuel, and wear particles from the engine. Depending on the size of these particles, they will penetrate our lungs when breathed in. Very small particles will even penetrate into our bloodstream. The very small particles are, therefore, the most hazardous to health.
This type of particulates are defined as PM10 (Particulate Matter 10). This means that the size of the measured average particles is 10 micrometres. You also currently see the term PM2.5 being used when particles that measure 2.5 micrometres are examined. A limit will also be set with regard to the particle quantity for inland navigation engines as is the case with Euro 6 trucks. This will, therefore, set a limit with regard to the very small particles that have little mass, but are still hazardous.
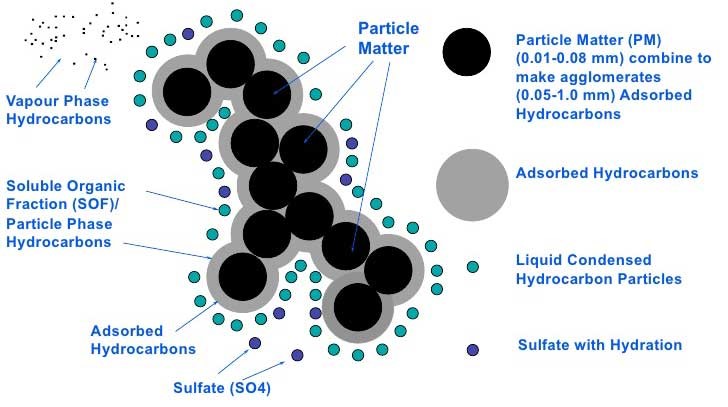
Particulate composition (Source: Twigg & Phillips 2009)
How does a diesel particulate filter system work?
Diesel particle filters are also called DPF of Soot Filters. The Xeamos diesel particulate filter systems mentioned on this website use high-quality and robust silicon carbide filter elements. These elements consist of a porous ceramic material where the channels are sealed alternately on one side. This ensures a very good filtering of exhaust gases. A thin layer is deposited in the channels that consists of particulates and ash. This thin layer provides the ultimate fine filtering of the soot particles.
The ash comes from the fuel and lubricant and stays behind in the channels while the particulates are burnt. This process is referred to as regeneration.
There are two methods to regenerate the particulates stored in the filter:
The first method is to combine the diesel oxidation catalyst (DOC) with a non-coated diesel particulate filter. This method is used quite often in the automotive industry and road transport. The diesel oxidation catalyst has a noble metal such as platinum or palladium and converts part of the nitrogen monoxide (NO) that can be found in the exhaust gases into nitrogen dioxide (NO2). This nitrogen dioxide is normally only formed in the atmosphere. The nitrogen dioxide is a reactive substance that oxidises (burns) the particulates (carbon and hydrocarbons) that are stored in the diesel particulate filter to form carbon dioxide (CO2) and water (H2O). The design of the diesel oxidation catalyst must be carefully aligned with the engine to ensure that not too much nitrogen dioxide can be formed. This can, in specific situations, lead to a yellow/brown discoloration of the exhaust gases. This method of regeneration is usually combined with an SCR system for this reason: to reduce the surplus nitrogen oxides.
A significant advantage of this method is that the regeneration of the diesel particulate filters already takes place at low temperatures (between 250-400 °C) and, in a way, they regenerate continuously. An important plus point is that the replacement and operational costs are significantly lower than with regard to the second method.
A limitation is that the diesel oxidation catalyst can only be used with ultra-low sulphur fuels such as EN 590. With sulphur-containing fuels such as DMA or DMX, the diesel oxidation catalyst would lose its effect prematurely because the sulphur adheres to the noble metal.
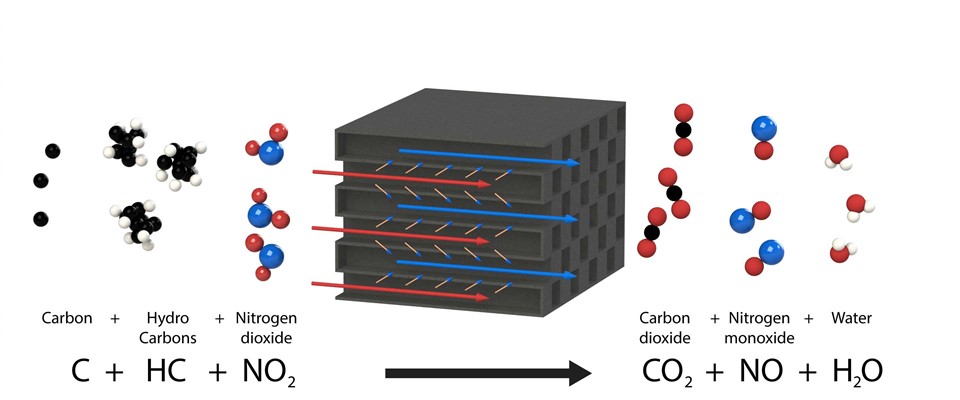
Chemical reactions of a NO2 regeneration diesel particulate filter
The second method for the regeneration of the diesel particulate filters is by providing them with a catalyst layer or coating. This layer ensures a reduction of the oxidation or burning temperature of the particulates stored in the filter. Without this coating, particulates would only burn as from approximately 600 °C. With the aid of the catalyst, the oxidation temperature can be reduced to approximately 350 – 400 °C. In most cases, a type of active regeneration is required because this temperature is higher than with the first method where the temperature of the exhaust gases is increased regularly using a burner or electric heating element to a value that is higher than 500 °C.
The advantage of this method is that specific types of coated diesel particulate filters (referred to as cDPFs) can withstand sulphur reasonably well. The high regeneration temperatures also ensure that the sulphur oxidises and does not stay behind in the filter.
A disadvantage is that the coating ages relatively quickly and ultimately the complete diesel particulate filters will have to be replaced. In addition to the fact that regeneration must take place actively frequently and at a higher temperature, the operational costs related to this method are higher than with the first method.
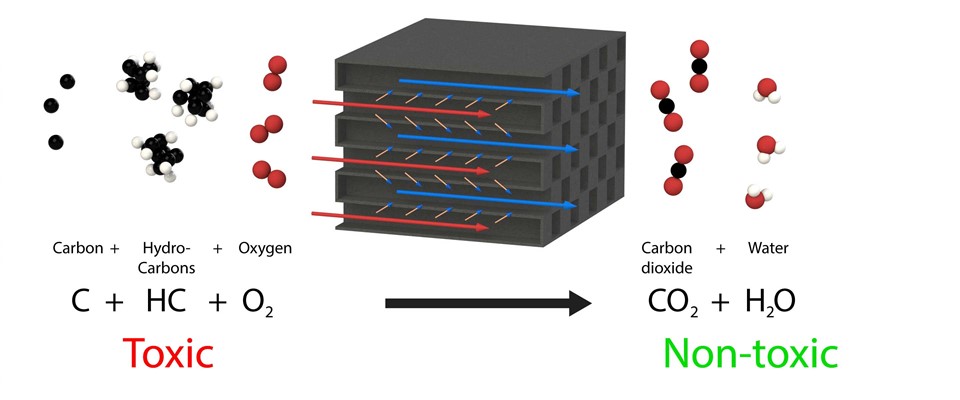
Chemical reactions of an O2 regeneration diesel particulate filter